Introduction: Despite the curative intent of the R-CHOP regimen in the first-line treatment of diffuse large B-cell lymphoma (DLBCL), 35-40% of patients who received R-CHOP will eventually succumb to their disease (Coiffier, et al. Blood 2010; Sarkozy and Sehn. Ann Lymphoma 2019). As such, improved treatments are needed. Mosunetuzumab (Mosun) is a T-cell-engaging bispecific antibody that redirects T cells to eliminate malignant B cells by binding to CD3 on T cells and CD20 on B cells. Mosun monotherapy has a manageable safety profile and promising efficacy, including durable complete responses (CR), in patients (pts) with relapsed and/or refractory (R/R) non-Hodgkin lymphoma (NHL) (Schuster, et al. ASH 2019). This is the first report describing the safety and efficacy of Mosun plus CHOP (M-CHOP) in pts with R/R NHL and newly diagnosed DLBCL in the ongoing GO40515 (NCT03677141) study.
Methods: Pts with R/R NHL and with newly diagnosed DLBCL received six 21-day cycles of M-CHOP. In Cycle (C) 1, Mosun was administered in step-up doses on Day (D) 1 (1mg), D8 (2mg), and D15 (13.5mg and 30mg in R/R NHL; 30mg in newly diagnosed DLBCL) to mitigate cytokine release syndrome (CRS). Full dose Mosun (C1D15 dose) was given on D1 of subsequent cycles in addition to CHOP. Interim and primary response assessments were obtained after C4 and C6, respectively. Primary prophylaxis with granulocyte colony-stimulating factor was mandatory for all pts. Pts with a partial response or stable disease at the end of C6 could continue Mosun monotherapy for up to 11 additional cycles. Response rates were based on the Lugano criteria (Cheson, et al. J Clin Oncol 2014).
Results: As of June 3, 2020, 43 pts had received M-CHOP: seven patients with R/R NHL, and 36 pts with newly diagnosed DLBCL. Pts with disease stage II-IV were enrolled, with a median IPI score of 3 (range: 2-4) and ECOG performance status between 0 and 2. Median age was 66 (range: 39-87) and 17 pts (42%) were female.
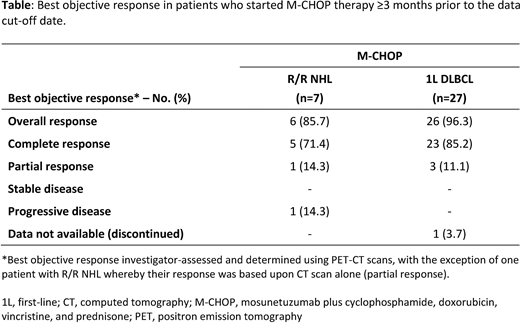
In pts with R/R NHL treated with M-CHOP (n=7), the overall response rate (ORR) was 86%, with 71% of pts achieving a CR. Twenty-seven out of 36 pts with previously untreated DLBCL started treatment at least three months prior to data cut-off date; in these pts the ORR was 96%, with a CR rate of 85% (Table).
Grade (Gr) ≥3 adverse events (AEs) occurred in 37 pts (86%) and serious AEs in 19 pts (44%). Two pts (29%) with R/R NHL experienced CRS (one with Gr 1 and one with Gr 2; ASTCT grading, Lee et al. Biol Blood Marrow Transplant 2019); one pt received tocilizumab. Nineteen pts (53%) with previously untreated DLBCL had CRS events (14 with Gr 1, five with Gr2); one pt received tocilizumab. No pts required vasopressors or high-flow oxygen. All CRS events occurred in C1, resolved without sequelae, and did not result in discontinuation or delay in treatment. No immune effector cell-associated neurotoxicity syndrome (ICANS) events were observed.
Neutropenia occurred in two pts with R/R NHL (29%; Gr 4 n=2) and 23 pts with newly diagnosed DLBCL (64%; Gr 3 n=3, Gr 4 n=20). Febrile neutropenia occurred in two pts (29%) with R/R NHL, and six pts (17%) with newly diagnosed DLBCL.
Gr 5 AEs, excluding disease progression, were reported in two pts: one due to Pneumocystis jirovecii pneumonia in a pt with R/R NHL, and one due to pneumonia in a pt with newly diagnosed DLBCL. All pts with R/R NHL have completed treatment. Among pts with newly diagnosed DLBCL, four have completed treatment and 29 remain on treatment; one pt died on-study (Gr 5 pneumonia), and two withdrew from the study treatment due to AEs (one due to treatment-unrelated esophageal perforation; one due to treatment-related pneumonitis).
Linear pharmacokinetics (PK) were observed for Mosun. No differences were seen in Mosun exposure for pts with R/R NHL and previously untreated DLBCL. Similar PK characteristics were seen with M-CHOP as with Mosun monotherapy, indicating no impact when co-administered with CHOP.
Conclusions: Preliminary data show that Mosun, a novel CD20/CD3 bispecific antibody, when combined with CHOP confers high response rates and a manageable safety profile in pts with R/R NHL and previously untreated DLBCL. End of treatment response rate data for pts with previously untreated DLBCL, and correlative studies of T-cell response, will be presented.
Phillips:Incyte: Consultancy, Other: travel expenses; AstraZeneca: Consultancy; Seattle Genetics: Consultancy; Bayer: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Pharmacyclics: Consultancy; Cardinal Health: Consultancy; BMS: Consultancy; Beigene: Consultancy; Karyopharm: Consultancy. Olszewski:TG Therapeutics: Research Funding; Adaptive Biotechnologies: Research Funding; Spectrum Pharmaceuticals: Research Funding; Genentech, Inc.: Research Funding. Munoz:Alexion: Consultancy; Portola: Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy; Bayer: Consultancy, Research Funding, Speakers Bureau; Kyowa: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Verastem: Speakers Bureau; Juno/Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Fosunkite: Consultancy; Innovent: Consultancy; Acrotech/Aurobindo: Speakers Bureau; AstraZeneca: Speakers Bureau; Genentech/Roche: Research Funding, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Merck: Research Funding; Incyte: Research Funding; Millenium: Research Funding. Kim:AstraZeneca: Consultancy; Novartis: Consultancy; Takeda: Consultancy; Sanofi: Consultancy; F. Hoffmann-La Roche Ltd/Genentech, Inc.: Consultancy; Voronoi: Consultancy; Boryung: Consultancy; AstraZeneca and Korea Health Industry Development Institute: Research Funding. Yoon:Celltrion: Honoraria; Samyang: Research Funding; Amgen, Chongkundang, Celgene, Astrazeneca: Consultancy. Greil:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; MSD Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; BMS/celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; Daiichi Sankyo, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; Astra zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodations, expenses, Research Funding. Westin:Novartis: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Curis: Consultancy, Research Funding; Morphosys: Consultancy, Research Funding; 47: Research Funding; Janssen: Consultancy, Research Funding; Amgen: Consultancy; Kite: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Jaeger:Novartis: Consultancy, Honoraria, Research Funding; Amgen: Honoraria; Infinity: Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; True North: Honoraria, Research Funding; Miltenyi: Consultancy, Honoraria; CDR Life AG: Consultancy, Research Funding; Karyopharm: Honoraria; Gilead: Honoraria, Research Funding; Takeda: Honoraria; AbbVie: Honoraria; F. Hoffmann-La Roche: Honoraria, Research Funding. Canales:Celgene, Gilead, iQone, Janssen, Karyopharm, Novartis, F. Hoffmann-La Roche, Sandoz: Honoraria; Janssen, F. Hoffmann-La Roche, Sandoz, Takeda: Speakers Bureau. Chen:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company; Bristol-Myer Squibb: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Janssen Pharmaceuticals: Current equity holder in publicly-traded company. Althaus:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. O'Hear:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche: Current equity holder in publicly-traded company. Negricea:F. Hoffmann-La Roche: Current Employment. Xie:F. Hoffmann-La Roche: Current Employment. McCord:Genentech, Inc.: Current Employment; F. Hoffman-La Roche: Current equity holder in publicly-traded company. Purev:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche: Current equity holder in publicly-traded company. Vallurupalli:Received Research funding to University of Kansas to conduct the ongoing GO40515 clinical trial for which the abstract is being submitted.: Research Funding; On Kite speaker Bureau but do not receive any honorarium.: Speakers Bureau.
Mosunetuzumab (RG7828; CD20-TDB) is a full-length, fully humanized immunoglobulin G1 (IgG1) bispecific antibody targeting both CD3 (on the surface of T cells) and CD20 (on the surface of B cells). Mosunetuzumab redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab is an investigational agent.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal